Soaps are sodium or potassium salts of combinations of fatty acids having cleansing action in water. Soaps are made from vegetable oils like sunflower oil, olive oil, coconut oil, vegetable fats, etc. The soap solution is a colloidal solution of micelles. The oil and dirt of the clothes or skin are absorbed by the hydrophobic hydrocarbon part of the soap by the mechanical action of rubbing. Layers from the dirty surfaces are converted into small globules.
Triglyceride (higher fatty acid of ester) + NaOH / KOH → Glycerol / glycerin + Soap
Some interesting facts about Soap
- Soap is a long chain of fatty acid with sodium and potassium, whereas detergent is long chain of ammonium salt of acid.
- Soap gives lather in soft water only, whereas detergent gives lather in water as well as hard water.
- Soap is biodegradable in nature while detergent in non-biodegradable in nature.
- Process of soap (R-COO-Na or R-COO-K) is called saponification, where R is Oleic acid (C17H33) & Linoleic acid (C17H31) etc.
Do it Yourself Experiment: Cleansing Action of Soap
Apparatus Required




Step wise Procedure
1.Pour 100ml of milk into white plate.
2. Add few drops of different food colors on the surface of milk. These colors represent the impurity with milk fat.
3. Add soap by cotton & tooth pick or finger by touching on the food colors on the surface of milk. Observe that colors move the fat practical, it is because colors attach with milk fat but fat doesn’t dissolve in water. So soap dissolve milk fat in water.
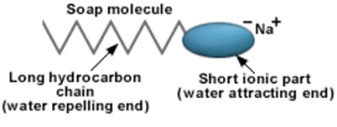
The hydrophilic end of soap interacts with water and hydrophobic end gets attached to the fat. By rinsing the solution, soap separates the particles of fat from the milk & makes it dissolved in water.
The dust particles remain trapped in micelles (which remain suspended) and are easily rinsed away with water.
Hence, soap micelles remove dirt by dissolving it in water.
Most dirt particles are oily in nature, and do not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic-end of soap interacts with water while the carbon chain interacts with oil.

